The quest for clean, sustainable energy has never been more critical. As the world grapples with the effects of climate change and the urgent need to reduce greenhouse gas emissions, proton exchange membrane fuel cells (PEMFC) have emerged as a beacon of hope. These innovative devices offer a zero-emission alternative to traditional fossil fuels, promising a future where our cars, homes, and industries are powered by clean hydrogen energy. Central to the function of PEMFC is the Membrane Electrode Assembly (MEA), the powerhouse where electrochemical reactions convert hydrogen into electricity.
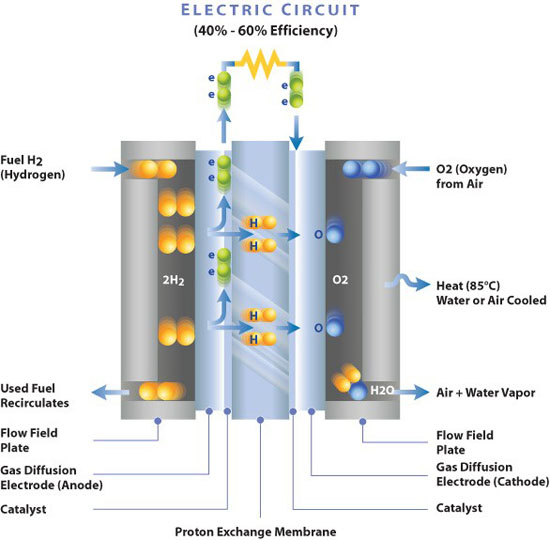
Recent advancements in MEA technology are breaking down the barriers that once hindered the widespread adoption of PEMFCs. Challenges like high costs, limited durability, and complex water management are being addressed through cutting-edge research and innovative manufacturing techniques. This comprehensive exploration delves into five key breakthroughs that are propelling PEM fuel cells to the forefront of clean energy solutions.
Why Are PEMFCs the Future of Clean Energy?
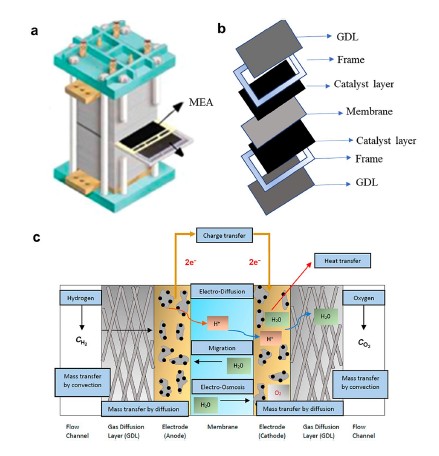
PEM fuel cells operate by harnessing the chemical energy of hydrogen and oxygen to produce electricity, with water and heat as the only byproducts. This clean energy conversion makes PEMFC an attractive option for reducing carbon footprints across various sectors. The MEA is the heart of the PEMFC, comprising multiple layers that facilitate the necessary reactions for energy production.
The potential applications of PEMFC are vast, ranging from powering electric vehicles to providing backup energy for homes and businesses. Their high efficiency and scalability make them suitable for both small-scale and large-scale energy needs. However, the journey toward mainstream adoption has been met with obstacles, primarily due to the expensive materials required and challenges in ensuring long-term durability.
1. Catalysts: Cutting Costs and Enhancing Efficiency
A significant hurdle in PEMFC technology has been the reliance on platinum-based catalysts. Platinum, being a rare and expensive metal, contributes substantially to the overall cost of fuel cells. These catalysts are essential for facilitating the oxygen reduction reaction (ORR) and the hydrogen oxidation reaction (HOR) within the MEA.
To overcome this, researchers are developing methods to reduce platinum content without compromising performance. One approach involves alloying platinum with more abundant transition metals like cobalt, nickel, or iron. This not only lowers the amount of platinum required but also enhances catalytic activity. The addition of these metals modifies the electronic structure of platinum, improving its interaction with oxygen molecules and accelerating reaction rates.
A notable breakthrough in this area is the creation of jagged platinum nanowires (J-Pt NWs). These nanowires exhibit a high electrochemical surface area of 118 m²/g and a mass activity of 13.6 A/mg Pt at 0.9V, surpassing traditional platinum catalysts by more than four times. The jagged structure increases the number of active sites available for reactions, boosting overall efficiency.
Exploring alternatives to platinum, non-platinum group metal (non-PGM) catalysts are gaining traction. Iron-nitrogen-carbon (Fe-N-C) catalysts have shown promise due to their lower cost and abundance. While they currently exhibit lower performance in acidic environments like those in PEMFC, ongoing research aims to enhance their activity and stability, potentially providing a viable and more sustainable alternative to platinum-based catalysts.
2. Membranes: Advancing Durability and High-Temperature Performance
The proton exchange membrane is critical for conducting protons from the anode to the cathode while preventing the mixing of hydrogen and oxygen gases. Traditional membranes like Nafion have limitations, including high cost and suboptimal performance at elevated temperatures and low humidity.
Advancements in membrane technology focus on developing materials that maintain high proton conductivity under a wider range of operating conditions. Short-side-chain (SSC) perfluorosulfonic acid (PFSA) membranes are one such innovation. These membranes offer higher ion exchange capacity (IEC) and better water retention compared to long-side-chain (LSC) variants. This results in improved performance, especially in low-humidity environments, and enhances the membrane’s durability by reducing degradation rates.
Another significant development is the introduction of hydrocarbon-based membranes, such as sulfonated polyether ether ketone (SPEEK) and polybenzimidazole (PBI). These materials are less expensive than fluorinated polymers and exhibit excellent proton conductivity. Phosphoric acid-doped PBI membranes, for example, enable PEMFC to operate at higher temperatures between 100–250°C. High-temperature operation improves reaction kinetics, enhances carbon monoxide tolerance in the fuel stream, and simplifies thermal and water management within the fuel cell system.
Researchers are also investigating composite membranes that combine the properties of different materials to achieve superior performance. Incorporating inorganic nanoparticles like silica or zirconia into polymer matrices can enhance mechanical strength, thermal stability, and proton conductivity. These composite membranes aim to extend the lifespan of PEMFCs while maintaining high efficiency.
3. Advanced Ink Formulations: Optimizing Catalyst Layer Architecture
The catalyst layer within the MEA is formed by depositing a thin film of catalyst ink onto a substrate. This ink is a complex mixture of catalyst particles, ionomer binders, solvents, and additives. The formulation and processing of this ink are crucial for achieving a uniform catalyst layer with optimal porosity and active surface area.
Recent innovations focus on improving the dispersion of catalyst particles within the ink to prevent agglomeration, which can reduce the number of active sites and hinder gas diffusion. Using solvents with appropriate dielectric constants enhances the interaction between the ionomer and catalyst particles, resulting in a more stable ink. Additionally, surfactants and dispersing agents are employed to maintain a homogeneous mixture.
Advanced techniques like ultrasonic dispersion and high-shear mixing are utilized to achieve better particle distribution. Freeze-drying methods have also been introduced to create catalyst layers with increased porosity. By sublimating the solvent, a porous structure is formed, which facilitates gas transport and increases catalyst utilization.
Transitioning to water-based inks is another significant development. Traditional ink formulations often rely on organic solvents, which pose environmental and safety concerns. Water-based inks are more environmentally friendly and reduce production costs. However, they require careful optimization to ensure that the catalyst particles and ionomers are well-dispersed and that the resulting films have the desired properties.
4. Gas Diffusion Layers: Enhancing Water and Gas Management
The gas diffusion layer (GDL) is essential for distributing reactant gases to the catalyst layer and facilitating the removal of water produced during operation. Effective GDL design ensures that the MEA remains properly hydrated without flooding, maintaining optimal proton conductivity and performance.
Innovations in GDL technology include the development of microporous layers (MPLs) composed of carbon black and hydrophobic polymers like polytetrafluoroethylene (PTFE). These layers are applied to the GDL to fine-tune its hydrophobicity and pore structure. By controlling these properties, the MPL enhances water management by promoting the removal of excess water while retaining enough moisture to keep the membrane hydrated.
Advanced GDLs with gradient porosity are being explored to further improve water management. These designs feature varying pore sizes throughout the thickness of the GDL, optimizing the balance between gas diffusion and water removal. Additionally, materials like carbon nanotubes and graphene are being investigated for GDL construction due to their excellent electrical conductivity and mechanical strength.
Innovative manufacturing techniques, such as electrospinning and 3D printing, allow for precise control over the GDL’s microstructure. These methods enable the creation of custom-designed GDLs tailored to specific operating conditions and performance requirements.
5. Manufacturing Innovations: Scaling Up with Quality and Efficiency
The commercial viability of PEMFCs depends not only on technological advancements but also on the ability to manufacture MEAs at scale with consistent quality and at a reduced cost. Manufacturing innovations are thus a critical aspect of bringing PEMFC technology to the mainstream market.
Roll-to-roll (R2R) processing is a manufacturing technique that enables continuous production of MEA components. This method integrates multiple steps, such as catalyst coating, membrane application, and layer assembly, into a seamless operation. R2R processing reduces production time and labor costs while enhancing uniformity and quality control.
Slot-die coating is a key technology in R2R manufacturing, allowing precise application of catalyst inks onto substrates. By adjusting parameters like coating gap, flow rate, and substrate speed, manufacturers can achieve uniform catalyst layers with controlled thickness and loading. This precision is vital for maximizing the performance and durability of the MEA.
The adoption of in-line monitoring and control systems further enhances manufacturing quality. Techniques like spectroscopy and machine vision are used to inspect the MEA components in real-time, detecting defects or inconsistencies immediately. This proactive approach minimizes waste and ensures that only MEAs meeting strict specifications proceed through the production line.
Artificial intelligence (AI) and machine learning are increasingly being integrated into manufacturing processes. AI algorithms analyze large datasets from production to optimize conditions, predict maintenance needs, and improve overall efficiency. For example, AI can optimize catalyst ink formulations by analyzing how different compositions affect performance, reducing the need for extensive experimental testing.
Automation and robotics are also playing a significant role in scaling up production. Automated assembly lines reduce human error and increase throughput. Collaborative robots, or “cobots,” work alongside human operators to handle delicate tasks, enhancing precision and safety.
The Broader Impact: PEMFCs in the Global Energy Landscape
The advancements in PEMFC technology extend beyond technical improvements; they have significant implications for global energy strategies and environmental policies. As countries commit to reducing carbon emissions under international agreements like the Paris Accord, PEMFCs offer a viable pathway to achieve these goals.
In the transportation sector, fuel cell electric vehicles (FCEVs) present an alternative to battery electric vehicles (BEVs), particularly for applications where long-range and quick refueling are essential. Heavy-duty vehicles like buses, trucks, and trains can benefit from PEMFCs due to their high energy density and scalability.
In stationary power generation, PEMFCs provide reliable backup power for critical infrastructure, such as hospitals and data centers. They can also be integrated into microgrids, enhancing energy security and resilience against grid disruptions.
Hydrogen production and distribution infrastructure are key enablers for the widespread adoption of PEMFCs. Efforts are underway globally to develop “green hydrogen” produced from renewable energy sources, further reducing the environmental impact. Investments in hydrogen refueling stations and pipelines are increasing, creating a supportive ecosystem for fuel cell technologies.
Overcoming Challenges: Collaboration and Standardization
While the progress is significant, challenges remain in achieving cost parity with traditional energy sources and ensuring long-term durability under various operating conditions. Collaboration among researchers, manufacturers, governments, and industry stakeholders is crucial to address these issues.
Standardization of materials, components, and testing methods can accelerate development by enabling more effective comparisons of technologies and sharing of best practices. International organizations are working to establish guidelines and certifications to ensure safety, performance, and interoperability of PEMFC systems.
Government policies and incentives play a pivotal role in fostering innovation and adoption. Subsidies, tax credits, and funding for research and development can lower barriers to entry for new companies and encourage existing industries to invest in fuel cell technologies.
Conclusion: Embracing a Sustainable Energy Future
The advancements in PEM fuel cell technology and MEA development mark a significant stride toward a sustainable energy future. By addressing key challenges in catalyst efficiency, membrane durability, catalyst layer optimization, and scalable manufacturing, PEMFCs are becoming more economically viable and practical for widespread use.
The integration of PEMFCs into various sectors promises to reduce carbon emissions substantially, enhance energy security, and stimulate economic growth through new industries and job creation. As technological innovations continue to emerge, supported by collaborative efforts and favorable policies, PEM fuel cells are poised to play a transformative role in how we produce and consume energy.
Embracing PEMFC technology is not just a scientific and industrial endeavor; it is a commitment to environmental stewardship and a sustainable future for generations to come. The road ahead is promising, and with continued dedication, the vision of a clean energy world powered by hydrogen fuel cells is within our reach.
Source: Mo, Shanyun, et al. “Recent advances on PEM fuel cells: from key materials to membrane electrode assembly.” Electrochemical energy reviews 6.1 (2023): 28.
https://doi.org/10.1007/s41918-023-00190-w
※This report has been prepared for general information purposes, and you may freely quote or reproduce the contents of this report for non-commercial purposes as long as the source of the contents is indicated. If you need to make a decision or need more information, please consult with CHEMiFORGE or an expert.