Background: Importance of Lithium and Magnesium Selectivity
Lithium is a crucial component in lithium-ion batteries, which power everything from electric vehicles to portable electronics, and the growing global demand for these batteries has made efficient lithium extraction a priority. However, one of the main challenges is separating lithium from magnesium, which is difficult because magnesium’s ion size is similar to that of lithium.
In a groundbreaking development that could revolutionize lithium production for batteries, researchers have fabricated a polyamide (PA) nanofiltration (NF) membrane that exhibits remarkable selectivity for separating lithium ions (Li+) from magnesium ions (Mg2+). The key to achieving this exceptional performance lies in the precise regulation of the membrane’s pore size during the interfacial polymerization (IP) process. By introducing an oil-soluble surfactant, dodecyl phosphate (DDP), the researchers created a monolayer at the oil/water interface, a technique they termed OSARIP (Oil-Soluble Surfactant Assisted Regulated Interfacial Polymerization).
Filtration Mechanism of Polyaminde Membrane
This membrane facilitated the controlled diffusion of the amine monomer (piperazine, PIP) into the organic phase, leading to a highly uniform IP reaction. Consequently, the resulting PA NF membrane exhibited an ultra-narrow pore size distribution, with pores ranging from 3.2 to 8.0 Angstroms (Å). Remarkably, almost all of these pores were smaller than the hydration diameter of Mg2+ ions (8.6 Å) but larger than the Stokes diameter of Li+ ions (4.8 Å).
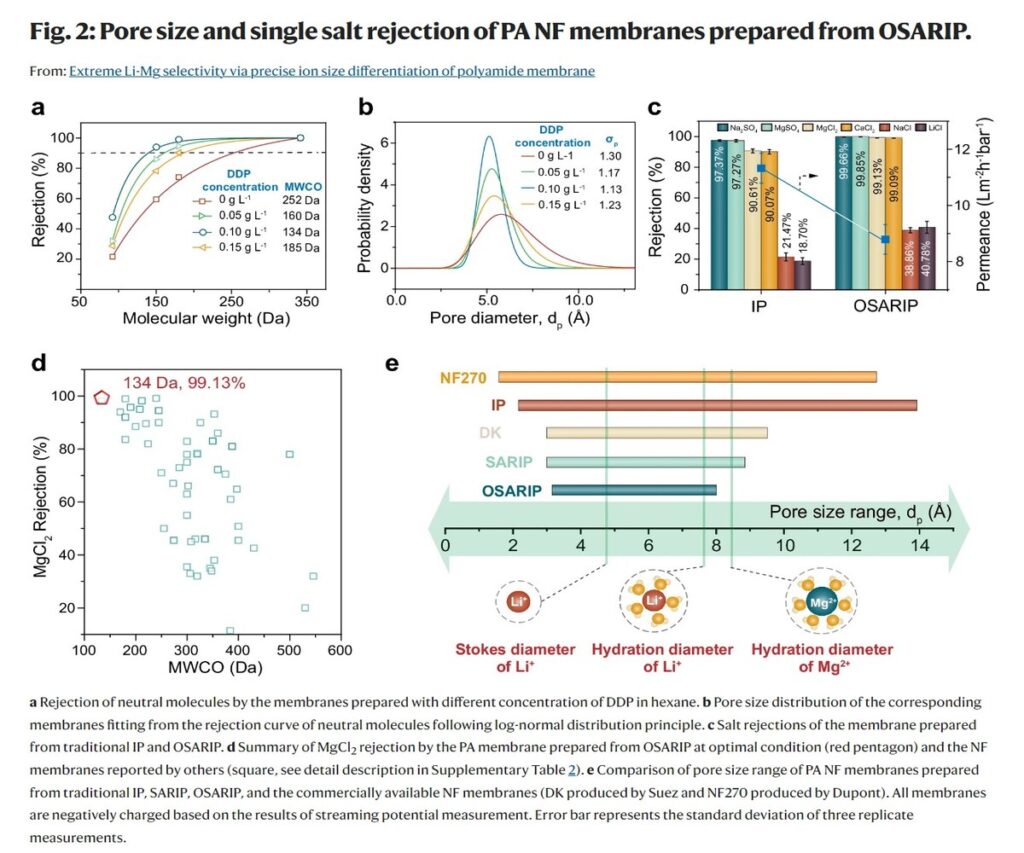
Through the sole effect of size sieving, the PA NF membrane achieved an exceptional Mg2+ rejection rate of over 99.9%, while maintaining a relatively low rejection of Li+. This resulted in an unprecedented Li+/Mg2+ selectivity, reaching values as high as 4147, which is one to two orders of magnitude higher than any currently reported pressure-driven membranes or even emerging microporous framework membranes.
The exceptional Li+/Mg2+ selectivity of the PA NF membrane enables the production of high-purity lithium filtrate through a single membrane filtration process. This breakthrough could revolutionize the existing technology for extracting lithium from brines, significantly simplifying the process and improving separation efficiency.
Theoretical analysis using the solution-diffusion-electromigration (SDEM) model revealed that the steric hindrance posed by the membrane’s pores had a limited effect on Li+ permeability but greatly reduced the permeability of Mg2+, resulting in the exponential increase of Li+/Mg2+ selectivity as the mean pore size decreased.
The researchers demonstrated the membrane’s exceptional performance by conducting separation tests using binary salt mixtures of lithium chloride (LiCl) and magnesium chloride (MgCl2), simulating brine conditions. Even in the presence of additional metal ions, such as sodium, potassium, and calcium, the PA NF membrane maintained remarkable Li+/Mg2+ selectivity, exceeding 200.
Manufacturing Method of Polyaminde Membrane
Delving into the details of membrane manufacturing, the researchers employed a two-step process. First, they prepared an aqueous solution containing 5 grams per liter (g/L) of piperazine (PIP) and a hexane solution containing 2 g/L of trimesoyl chloride (TMC) and a specific concentration of dodecyl phosphate (DDP). These solutions were heated to 35°C and maintained at this temperature.
To fabricate the PA NF membrane via the OSARIP process, the surface of a polyethersulfone (PES) ultrafiltration membrane was first contacted with the PIP aqueous solution for 60 seconds. Any visible aqueous drops on the membrane surface were gently wiped away. Subsequently, the TMC hexane solution containing DDP was poured onto the surface of the PES membrane and held horizontally for 30 seconds, allowing the IP reaction to occur.
The membrane was then washed with pure hexane for 30 seconds to remove any unreacted TMC. After curing in an oven at 60°C for 30 minutes and washing with pure water, the PA NF membrane was stored in water at 4°C for further use.
The presence of the DDP monolayer at the oil/water interface played a crucial role in regulating the IP reaction. The amphiphilic nature of DDP enabled the formation of an assembled monolayer, which adsorbed and accumulated PIP molecules near the interface through electrostatic interactions. This facilitated the controlled diffusion of PIP monomers into the organic phase, promoting a spatially uniform and continuous IP reaction.
In contrast, during the traditional IP process, the diffusion of PIP monomers is slow and random, leading to spatial and temporal discontinuities in the PA layer formation. This results in chemical and structural heterogeneity, contributing to a wide pore size distribution.
Filtration Performance of Polyaminde Membrane
The researchers employed various characterization techniques to analyze the membrane’s structure and performance. X-ray photoelectron spectroscopy (XPS) revealed a significantly higher crosslinking degree of 88.6% in the PA selective layer prepared from OSARIP, compared to 74.7% for the traditional IP process. Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) images showed a thinner PA selective layer (approximately 55 nm) with small convex spots on the surface for the OSARIP membrane.
Notably, the rejection of neutral molecules by the OSARIP membranes decreased with increasing DDP concentration, reaching a minimum molecular weight cut-off (MWCO) of 134 Daltons at the critical DDP concentration of 0.1 g/L. This ultra-low MWCO value highlights the exceptional pore size control achieved through the OSARIP process.
The separation performance of the PA NF membranes for Li+/Mg2+ was evaluated using binary salt mixtures of LiCl and MgCl2, mimicking brine conditions. The membrane prepared from OSARIP at 0.1 g/L DDP exhibited a remarkable Mg2+ rejection of 99.82% and a negative Li+ rejection of -48.35%, corresponding to a Li+/Mg2+ selectivity of 828. In contrast, the traditional IP membrane showed a lower Mg2+ rejection of 92.56% and a Li+/Mg2+ selectivity of only 16.
As the Mg2+/Li+ mass ratio in the binary salt mixture increased from 10:1 to 60:1, the Mg2+ rejection gradually increased to 99.96%, while the Li+ rejection became more negative, reaching -47.14%. This resulted in an astonishing Li+/Mg2+ selectivity of up to 4147, surpassing all previously reported pressure-driven membranes and even microporous framework materials.
The researchers further demonstrated the membrane’s exceptional performance under conditions mimicking actual salt lake brines, containing additional metal ions like sodium, potassium, and calcium. Even in these complex scenarios, the PA NF membrane maintained remarkable Li+/Mg2+ selectivity, exceeding 200.
This breakthrough in membrane technology has the potential to revolutionize the lithium extraction process from brines, significantly simplifying the process and improving separation efficiency. By enabling the production of high-purity lithium filtrate through a single membrane filtration step, this innovation could pave the way for more sustainable and cost-effective lithium production, supporting the growing demand for lithium-ion batteries and the global transition towards renewable energy sources.
Source: Peng, Quan, et al. “Extreme Li-Mg selectivity via precise ion size differentiation of polyamide membrane.” Nature Communications 15.1 (2024): 2505.
https://doi.org/10.1038/s41467-024-46887-4
※ This report has been compiled for the purpose of providing general information. It is based on data gathered by CHEMiFORGE. Should you have any inquiries or need to make decisions based on this report, it is advisable to consult with a CHEMiFORGE.