Introduction: Methanol to Hydrocarbons (MTH)
The pursuit of sustainable chemical processes has led researchers to explore innovative pathways for converting abundant resources into valuable chemicals. Among these, the conversion of methanol to hydrocarbons (MTH) stands out due to its potential to leverage methanol—a simple, widely producible molecule—into more complex hydrocarbons. Traditional catalysts used in this process, zeolites, face a significant challenge: deactivation through coke deposition.
Zeolite catalysts, while highly effective in the early stages of the MTH conversion, suffer from rapid deactivation, a phenomenon largely attributed to the accumulation of carbonaceous deposits known as coke. These deposits block the active sites of the catalyst, reducing its activity and necessitating regular, often costly, regeneration processes. The search for a solution has led to the exploration of metal additives, with recent attention turning to the unique properties of liquid metals such as Gallium (Ga) for their potential to enhance catalyst stability.
Mechanisms Behind the Enhanced Stability: Liquid Metal Catalyst
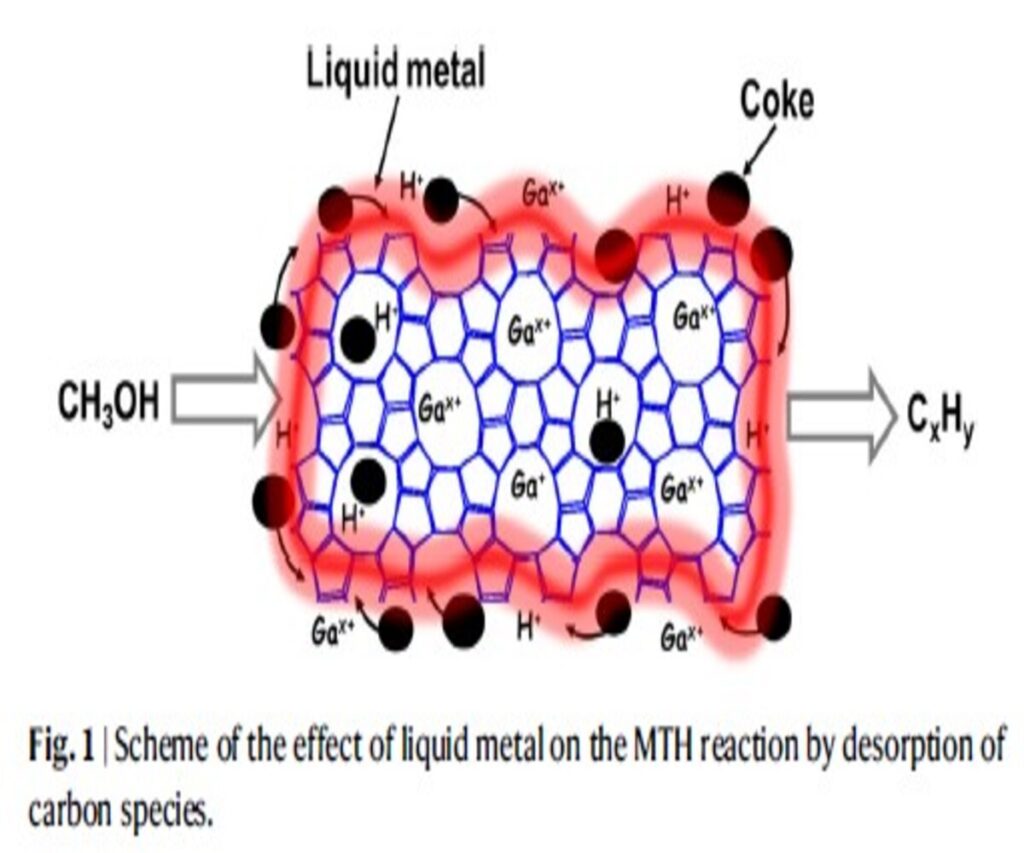
The foundation of this innovative breakthrough lies in the complex interplay between liquid gallium (Ga) and the microscopic structure of zeolite crystals. This interaction fundamentally alters the zeolite, amplifying its capability to release coke deposits from its acid sites. Consequently, this maintains the zeolite’s catalytic activity over longer durations. The study explores a variety of strategies to sustain stable methanol conversion, positioning liquid metals as a revolutionary solution. By diminishing coke deposition and promoting the removal of carbonaceous compounds, liquid metals like Ga are paving the way for a significant shift in catalytic processes.
Experimental Insights and Methodologies
The experimental approach is built on detailed methodological planning, from the initial preparation of NH4-ZSM-5 to the deliberate introduction of Ga into the ZSM-5 structure. The catalytic evaluations, executed under tightly regulated conditions, revealed the improved stability and performance of the Ga-enhanced ZSM-5 catalyst in the MTH conversion process. Complementing these practical observations, density functional theory (DFT) calculations provided deep insights into the molecular dynamics involved, clarifying the cooperative interaction between Ga clusters and zeolite acid sites in preventing catalyst deactivation.
Comprehensive Results and Analysis
The experimental outcomes were striking: Ga-enriched ZSM-5 demonstrated a markedly increased stability, with its operational lifespan extending up to about 14 times longer than that of the standard ZSM-5 catalyst. This advancement is credited to the unique synergy between Ga and the zeolite framework, which effectively reduces coke formation and aids in the expulsion of carbonaceous materials. Moreover, thorough analyses of the catalyst, both pre- and post-reaction, shed light on the operational mechanism, showing how Ga not only embellishes the zeolite’s surface but also modifies its acid properties, thus bolstering both stability and the efficacy of conversion.
Breakthrough Research on Liquid Metal Catalyst Promoters
In an avant-garde exploration, scientists have leveraged the distinct characteristics of low-melting-point metals, notably Gallium (Ga), to bring about a transformative improvement in the MTH conversion process. The discovery that the physical amalgamation of ZSM-5 zeolite with liquid gallium could significantly prolong the catalyst’s functional life by as much as approximately 14 times in comparison to its traditional equivalents signifies a pivotal advancement. This innovative technique not only counteracts swift deactivation but also unlocks new paths toward the realization of sustainable and more efficient hydrocarbon synthesis.
The innovation originated from a unique method to adjust the ZSM-5 zeolite, frequently employed in MTH conversion, with liquid Ga. The researchers crafted the modified catalyst by manually combining ZSM-5 with Ga under precise conditions to assure the metal’s thorough integration. This methodology encompassed the calcination of NH4-ZSM-5 to procure ZSM-5, succeeded by its saturation with Ga to fabricate the aimed Ga-altered ZSM-5 catalyst.
The assessment of catalytic efficiency was conducted in a controlled laboratory setting, mirroring the MTH conversion procedure. These experiments were designed to assess the resilience and productivity of Ga-altered ZSM-5 in contrast to conventional ZSM-5 catalysts. Essential parameters, including temperature, methanol flow rate, and catalyst formulation, were rigorously managed to confirm the dependability of the findings.
Implications and Future Directions
This groundbreaking study not only demonstrates a viable method to significantly extend the lifetime of zeolite catalysts in MTH conversion but also opens new avenues for the application of liquid metals in catalysis. The findings suggest potential for broader use in various chemical conversions and processes, promising to revolutionize the field of catalysis with sustainable, efficient solutions.
Source: Zhou, Yong, et al. “Liquid metals for boosting stability of zeolite catalysts in the conversion of methanol to hydrocarbons.” Nature Communications 15.1 (2024): 2228.
https://doi.org/10.1038/s41467-024-46232-9
※ This report has been compiled for the purpose of providing general information. It is based on data gathered by CHEMiFORGE. Should you have any inquiries or need to make decisions based on this report, it is advisable to consult with a CHEMiFORGE.