Introduction: Need to Dry Process for Lithium Ion Battery (LIB)
Lithium Ion Batteries(LIB) are widely used as a power source for electrified vehicles such as electric vehicles and plug-in hybrid vehicles. As demand for lithium-ion batteries increases rapidly, reducing the material and manufacturing costs and environmental impact of these batteries has become a critical issue for the battery industry as a whole. At the same time, the widespread adoption of electric vehicles in the future with much higher capacity and power It depends on the development of technology to implement batteries.

The conventional lithium ion battery electrode manufacturing process is to manufacture an electrode slurry by mixing active materials (AM), conductive and binding additives (CBA), etc. in a solvent, coating the electrode slurry on a metal foil, and then drying the solvent. This process is a simple way to manufacture electrodes, but it consumes a lot of energy because it requires a high-temperature dryer to evaporate the solvent.
In particular, N-methyl-2-pyrrolidone (NMP), a solvent commonly used in slurries, is highly toxic and requires recovery and treatment. In the slurry-based wet electrode manufacturing process, drying and recovery of solvent accounts for approximately 20% of the total cost and approximately 50% of total energy consumption, creating a need for a process that shortens or eliminates the drying and recovery process or reduces energy consumption. It is increasing.
In response to these demands, dry process of electrode that do not use solvents have recently been in the spotlight. The dry process does not require solvents from the electrode material mixing stage to the printing and immobilization stages, so there is no need to dry or recover the solvent, which has the advantage of significantly reducing material costs and energy consumption in electrode manufacturing.
Therefore, in order to implement the dry process, Japanese scientists mixed electrode materials with a high-speed mixer to produce an electrode powder mixture (Dry Mixing), electrostatic screen coating the electrode powder mixture (Electrostatic Screen Printing), and coated electrode powder(Hot Roller Pressing).
A three-step process was used: hot roller pressing to fix the mixture. In order to evaluate the effect on the structure and electrochemical performance of the electrode manufactured in the dry electrode manufacturing process, the structure and electrochemical performance of the electrode were evaluated by varying the speed of the high-speed mixer.
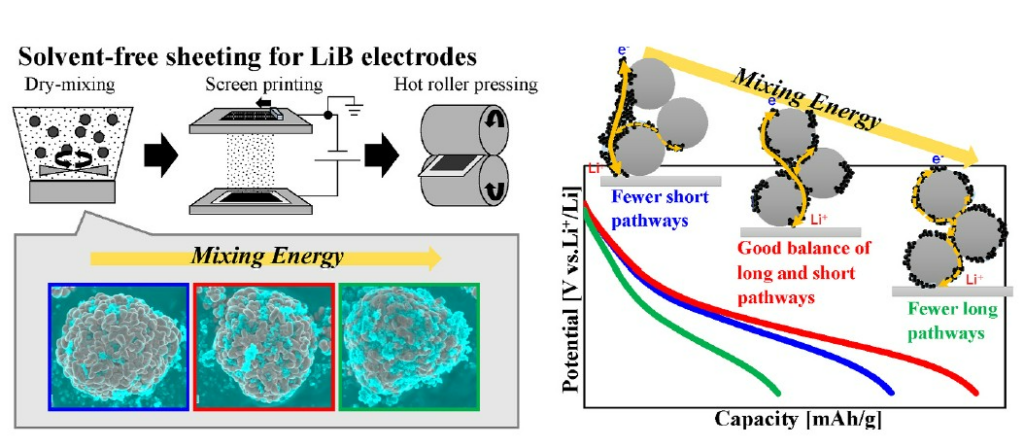
It was confirmed that as the rotation speed of the high-speed mixer increases, the distribution of the bonding additive (CBA) at the electrode becomes more uniform and the amount of fine particles increases. However, the change in distribution of particles and bonding additive (CBA) over time does not differ significantly.
It was confirmed that And comparing the electrochemical performance, the electrode with low rotation speed had low electrode active material (AM) particle coverage of the bonding additive (CBA), and the electrode with high rotation speed had high coverage of electrode active material (AM) particles with bonding additive (CBA). confirmed that It was confirmed that the coverage of the conductive and binder additive (CBA) to the electrode active material (AM) can be adjusted according to the rotation speed of the mixer.
Discharge capacity retention improved as rotation speed increased, but the internal resistance of the electrode increased. This result suggests that as the rotation speed increased, the Longer Conductive Pathways of the bonding additive (CBA) in the electrode were destroyed and the internal resistance increased.
Conclusion: Dry Process for Lithium Ion Battery (LIB)
In the dry process, electrode powder mixing conditions have a significant impact on electrode structure and electrochemical performance. It is expected that the electrochemical performance of the electrode can be improved through electrode structure control by optimizing the electrode powder mixing process in the dry process and is expected to contribute to the practical use of the dry process electrode manufacturing process.
In the dry process for lithium ion batteries, the conditions under which the electrode powder constituents are mixed together play a pivotal role in determining the resultant electrode structure and, consequently, its electrochemical performance characteristics in the dry process. The intimate blending of the active material particles, conductive additives, and polymeric binders is a critical step that governs the homogeneity, porosity, and overall architecture of the electrode composite in the dry process, all of which exert a profound influence on the electrochemical behavior and energy storage capabilities of the final electrode in the dry process.
It is widely recognized that optimizing the electrode powder mixing process in the dry process offers a promising avenue for enhancing the electrochemical performance of the electrode through deliberate control and tailoring of its structural attributes in the dry process. By carefully tuning parameters such as mixing time, shear forces, and the sequence of component addition in the dry process, it becomes possible to engineer the electrode morphology and achieve desirable features like uniform particle distribution, optimal pore network formation, and effective electronic and ionic conduction pathways in the dry process.
Consequently, there is a well-founded expectation that the electrochemical performance metrics, including capacity retention, rate capability, and cycle life, can be markedly improved through judicious optimization of the electrode powder mixing conditions in the dry process. This presents an opportunity to unlock the full potential of the dry process electrode manufacturing process, which has garnered significant interest due to its inherent advantages, such as reduced environmental impact, higher energy efficiency, and improved cost-effectiveness compared to conventional solvent-based electrode fabrication techniques.
Source: Yonaga, Ayaka, et al. “Effects of dry powder mixing on electrochemical performance of lithium-ion battery electrode using solvent-free dry forming process.” Journal of Power Sources 581 (2023): 233466.
https://doi.org/10.1016/j.jpowsour.2023.233466
※ This report has been compiled for the purpose of providing general information. It is based on data gathered by CHEMiFORGE. Should you have any inquiries or need to make decisions based on this report, it is advisable to consult with a CHEMiFORGE.