Introduction – Barium Iron Oxides
Scientists studied two unknown ternary oxides, Ba3Fe2O6 and Ba5Fe2O8. The primary objective is to assess their efficacy in two critical environmental applications: the capture of carbon dioxide (CO2) and their role in chemical looping oxygen uncoupling (CLOU).
The study is driven by the urgent need to develop effective technologies for reducing CO2 emissions, a major contributor to climate change. By investigating these specific oxides, research can advance the broader field of environmental engineering, particularly developing sustainable and efficient methods for greenhouse gas reduction. Scientists studied two unknown ternary oxides, Ba3Fe2O6 and Ba5Fe2O8.
The primary objective is to assess their efficacy in two critical environmental applications: the capture of carbon dioxide (CO2) and their role in chemical looping oxygen uncoupling (CLOU). The study is driven by the urgent need to develop effective technologies for reducing CO2 emissions, a major contributor to climate change. By investigating these specific oxides, research can advance the broader field of environmental engineering, particularly developing sustainable and efficient methods for greenhouse gas reduction.
Scientists studied two unknown ternary oxides, Ba3Fe2O6 and Ba5Fe2O8. The primary objective is to assess their efficacy in two critical environmental applications: the capture of carbon dioxide (CO2) and their role in chemical looping oxygen uncoupling (CLOU). The study is driven by the urgent need to develop effective technologies for reducing CO2 emissions, a major contributor to climate change.
By investigating these specific oxides, research can advance the broader field of environmental engineering, particularly developing sustainable and efficient methods for greenhouse gas reduction.

Barium Iron Oxides – CO2 Capture Capabilities
The research meticulously examines the CO2 capture abilities of Ba3Fe2O6 and Ba5Fe2O8. It was observed that both oxides could cyclically capture CO2 at temperatures above 800°C. Notably, Ba5Fe2O8 demonstrated a more robust CO2 capture performance compared to Ba3Fe2O6, exhibiting consistent gravimetric CO2 uptake capacities of 13.39 wt% at 1000°C over multiple cycles. This finding is significant as it highlights the potential of Ba5Fe2O8 in efficiently capturing CO2 in high-temperature industrial environments.
Barium Iron Oxides – Performance in Chemical Looping Oxygen Uncoupling (CLOU)
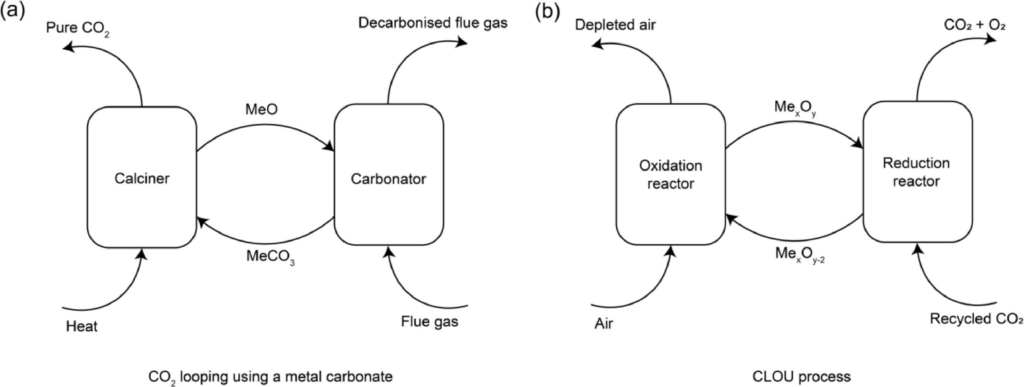
The study extends to evaluate the performance of these oxides in CLOU processes. Here, Ba3Fe2O6 stands out with its excellent recyclability and satisfactory CLOU activity, maintaining stability and effectiveness over temperature swings between 550°C and 950°C. This contrasts with Ba5Fe2O8, whose strong affinity for CO2, while beneficial for capture, renders it less suitable for CLOU applications.
Conclusion
We present a comprehensive and detailed investigation of the properties and potential applications of Ba3Fe2O6 and Ba5Fe2O8 in CO2 capture and CLOU.The findings reveal that while Ba5Fe2O8 shows superior performance in CO2 capture due to its higher uptake capacity, Ba3Fe2O6 is more suited for CLOU applications owing to its excellent recyclability and stable performance under thermal cycling.
We expect that these insights from the scientists will not only contribute to the scientific understanding of ternary oxides in environmental applications, but also pave the way for further research and development of sustainable CO2 management strategies.
We present a comprehensive and detailed investigation of the properties and potential applications of Ba3Fe2O6 and Ba5Fe2O8 in CO2 capture and CLOU.The findings reveal that while Ba5Fe2O8 shows superior performance in CO2 capture due to its higher uptake capacity, Ba3Fe2O6 is more suited for CLOU applications owing to its excellent recyclability and stable performance under thermal cycling.
We expect that these insights from the scientists will not only contribute to the scientific understanding of ternary oxides in environmental applications, but also pave the way for further research and development of sustainable CO2 management strategies. We present a comprehensive and detailed investigation of the properties and potential applications of Ba3Fe2O6 and Ba5Fe2O8 in CO2 capture and CLOU.The findings reveal that while Ba5Fe2O8 shows superior performance in CO2 capture due to its higher uptake capacity, Ba3Fe2O6 is more suited for CLOU applications owing to its excellent recyclability and stable performance under thermal cycling.
We expect that these insights from the scientists will not only contribute to the scientific understanding of ternary oxides in environmental applications, but also pave the way for further research and development of sustainable CO2 management strategies.
Source: Saqline, Syed, et al. “w” Applications in Energy and Combustion Science (2023): 100238.
https://doi.org/10.1016/j.jaecs.2023.100238
※ This report has been compiled for the purpose of providing general information. It is based on data gathered by CHEMiFORGE. Should you have any inquiries or need to make decisions based on this report, it is advisable to consult with a CHEMiFORGE.