Introduction: Ammonia Synthesis Costs and Energy Consumption
Ammonia (NH3) is the commercial fertilizer with the highest nitrogen (N2) content and has played a major role in freeing the world from hunger over the past 100 years. Recently, ammonia has received attention as a potential hydrogen (H2) carrier that could help reduce carbon emissions to practically zero in the green hydrogen economy.
The synthesis of ammonia, which relies heavily on high pressure and temperature, is manufactured using the traditional Haber Bosch process and is notorious for its energy-intensive nature. Therefore, the topic that stands out like a signal of innovation in the field of sustainable energy and eco-friendly chemical processes is the ammonia synthesis process.
Iron catalysts are widely used for ammonia synthesis. Iron is the most widely used catalyst for synthesis because it has an excellent ability to promote the reaction between nitrogen and hydrogen. The activity of ammonia iron catalyst depends on the size, shape, surface area, etc. of the iron particles. In general, the smaller the iron particle size and the larger the surface area, the higher the activity. And the ammonia iron catalyst is used at high temperature (400-500°C) and high pressure (200-300 atmospheres), and under these conditions, the ammonia synthesis reaction occurs most actively, making it the most important technology for synthesis.
The exothermic nature of synthesis (N2 + 3H2 = 2NH3, −92.44 kJmol−1) favors low temperatures, but high temperatures are required to cleave stable nitrogen bonds. This slows down the overall reaction rate and lowers the ammonia concentration. To increase the rate of ammonia synthesis, potassium oxide (K2O) is often used as an accelerator in industry, which can accelerate nitrogen adsorption and dissociation on the surface of the iron (Fe) catalyst.
However, because oxygen in potassium oxide interferes with iron when adsorbing nitrogen, an iron catalyst with pure metallic potassium (K) added is more effective in ammonia synthesis. However, pure metallic potassium is not thermally stable and is prone to evaporation above 400 degrees.
Recently, researchers in South Korea have solved this problem by developing a groundbreaking process for ammonia synthesis that is environmentally friendly and exploits the potential of volatile potassium in an interesting method called mechanochemistry.
Using a mechanochemical ball milling process, synthesis was developed by sequentially injecting iron powder with nitrogen and hydrogen under mild conditions (45°C, 1 bar), and the performance of the iron catalyst was improved by using pure metallic potassium. studied.
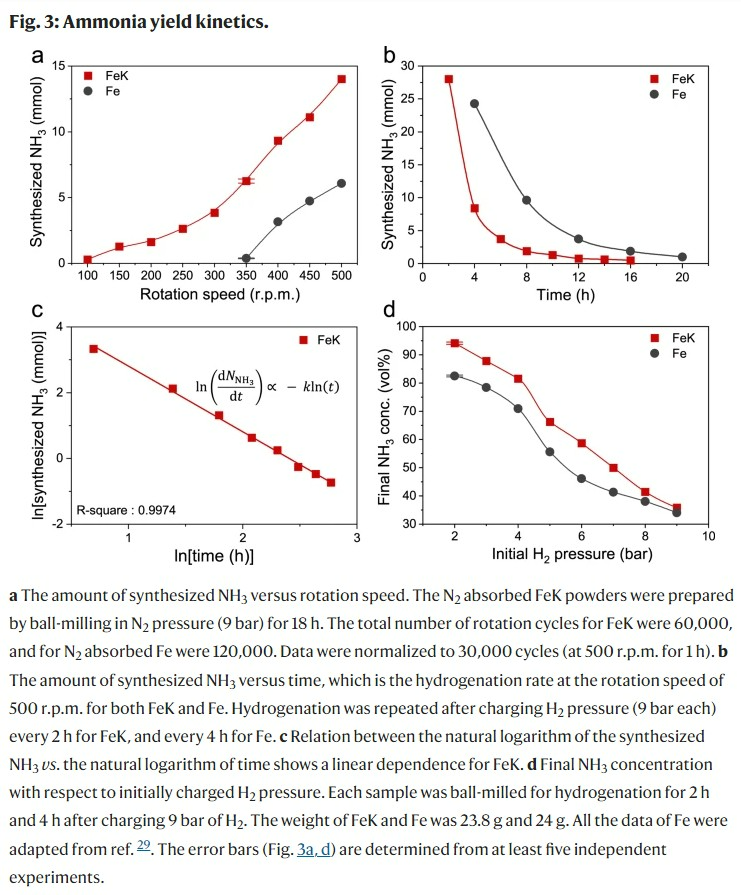
As a result, the synthesis rate was five times higher than when only an iron catalyst was used, and ammonia synthesis was successful even at a temperature and pressure slightly higher than room temperature, securing a low-cost, high-productivity technology.
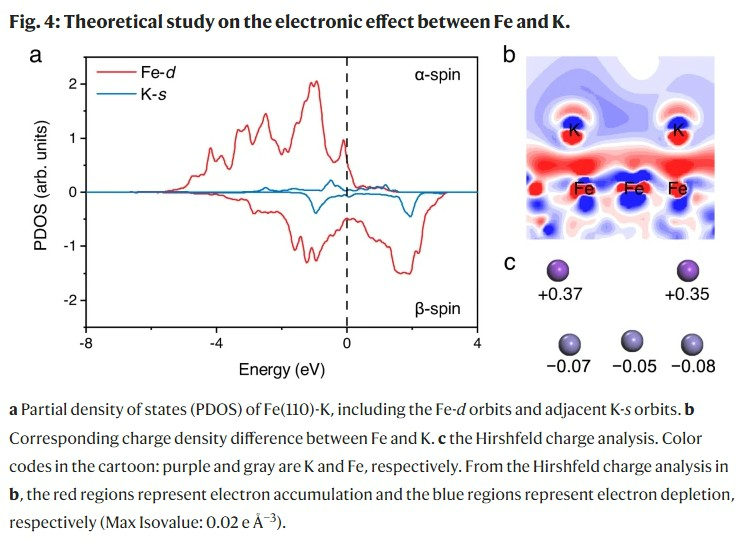
Additionally, by using Partial Density of States (PDOS) to study the electronic changes between iron and potassium, it was confirmed that there is a chemical interaction rather than weak physical adsorption between iron and potassium. This theoretical study revealed that the electrons accumulated in the iron atom as the potassium atom loses electrons help break the nitrogen bond, and the electron depletion of potassium allows the iron atom to easily adsorb hydrogen.
Conclusion: Ammonia Synthesis with Mechanochemistry
Mechanochemistry is a technology that promotes chemical reactions using mechanical force. This technology is known to consume less energy and be more environmentally friendly than traditional chemical reactions. Ammonia synthesis using mechanochemistry using potassium metal and iron catalysts has been shown to reduce energy consumption and environmental pollution compared to existing ammonia synthesis methods, and is evaluated to have the potential to enable economical chemical synthesis.
Source: Kim, Jong-Hoon, et al. “Achieving volatile potassium promoted ammonia synthesis via mechanochemistry.” Nature Communications 14.1 (2023): 2319.
https://doi.org/10.1038/s41467-023-38050-2
※ This report has been compiled for the purpose of providing general information. It is based on data gathered by CHEMiFORGE. Should you have any inquiries or need to make decisions based on this report, it is advisable to consult with a CHEMiFORGE.